Document Type : Original Research Article
Authors
- Sakshi Duklan 1
- Supriyo Saha 1
- Vikash Jakhmola 1
- Nidhi Gairola 1
- Pallavi Pandey 1
- Mahipal Singh 2
- Sarkar Mohammad Abe Kawsar 3
1 Department of Pharmaceutical Chemistry, Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Premnagar, Dehradun-248007, Uttarakhand, India
2 School of Agriculture, Uttaranchal University, Dehradun, Uttarakhand, India
3 Laboratory of Carbohydrate and Nucleoside Chemistry, Department of Chemistry, Faculty of Science, University of Chittagong, Chittagong 4331, Bangladesh
Abstract
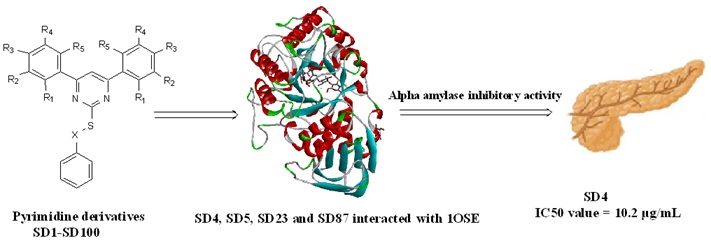
A newer generation pyrimidine derivatives were designed, synthesized, and evaluated in vitro alpha amylase and bacterial growth inhibitor. The molecules' design fully depended upon the structural features of previously pyrimidine derivatives. Then all the designed molecules (SD1-SD100) were docked with 1OSE pig pancreatic alpha-amylase isoenzyme. S-[4-(2-hydroxyphenyl)-6-phenylpyrimidin-2-yl] benzenecarbothioate, S-(4,6-diphenylpyrimidin-2-yl) benzenecarbothioate, S-[4-(4-hydroxy-3-methoxyphenyl)-6-phenylpyrimidin-2-yl] benzenecarbothioate, and S-[4,6-bis(4-hydroxyphenyl)pyrimidin-2-yl] benzenecarbothioate showed good docking interaction scores, as compared to acarbose. The interacting residues of the synthesized molecules and 1OSE showed similar amino acid lining as present in the active site. The synthetic procedure of the molecules was divided into two steps such as synthesis of chalcone derivative using aromatic aldehyde and acetophenone, reaction between chalcone and thiourea to form substituted pyrimidine-2-thiol, then finally substituted pyrimidine-2-thiol and benzoyl chloride reacted in presence of glacial acetic acid to obtain the best docked molecules. All the molecules show characteristic peaks in FTIR, 1H-NMR and Mass spectrometric data. Among all the synthesized molecules S-[4-(2-hydroxyphenyl)-6-phenylpyrimidin-2-yl] benzenecarbothioate showed best in vitro alpha amylase inhibition activity. Also, all synthesized molecules showed moderate to good antibacterial activities.
Graphical Abstract
Keywords
Main Subjects
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company